
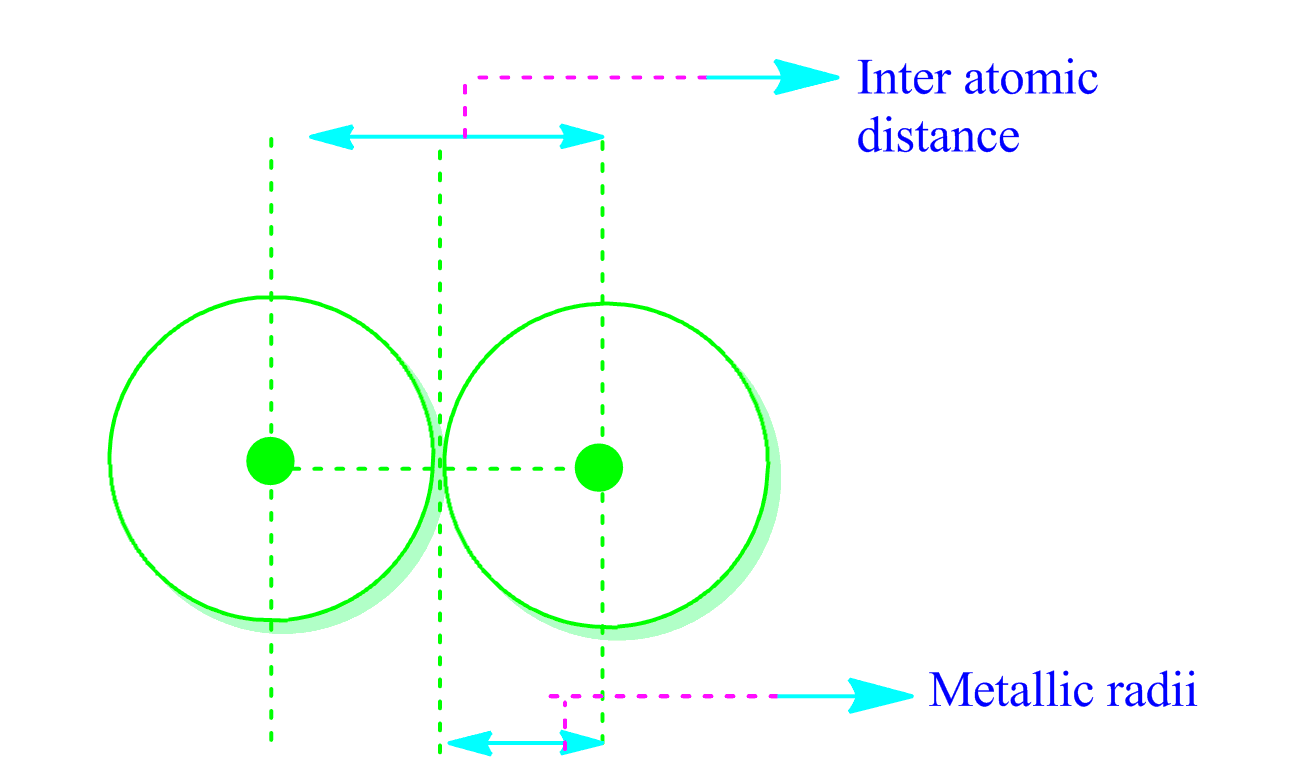
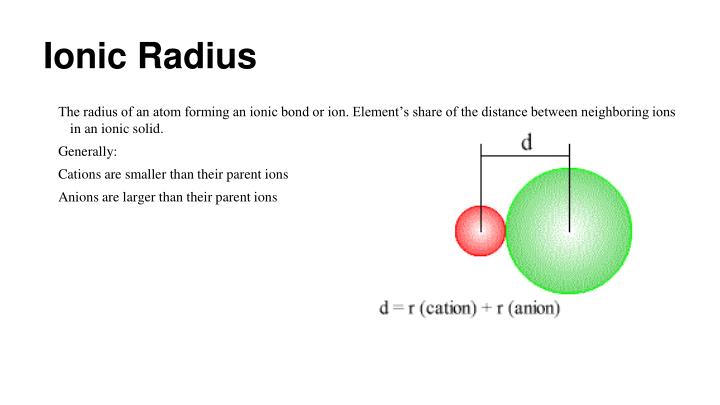

Many applications of the model in physics, materials science, chemistry, mineralogy, soil science, surface science, and molecular biology are reviewed. It emphasizes the separate roles of the constraints of chemistry and of three-dimensional space by analysing the chemistry of solids. This is the first book to explore in depth the theoretical basis of the model and to show how it can be applied to synthetic and solution chemistry. Unlike other models of inorganic chemical bonding, the bond valence model is simple, intuitive, and predictive, and can be used for analysing crystal structures and the conceptual modelling of local as well as extended structures. Recent improvements in crystal structure determination have allowed the model to become more quantitative. This book describes the bond valence model, a description of acid-base bonding which is becoming increasingly popular particularly in fields such as materials science and mineralogy where solid state inorganic chemistry is important. AllĪrticle content, except where otherwise noted, is licensed under a Creative Commons Some of who have found values of rvdW close to. To the present methodology is considerably larger than rvdW obtained by other investigators, To the two configurations 5dn+16s1 and 5dn6s2. From 71Lu to 80Hg the radius rear also involves two sequences, corresponding The sphere defined by rear contains 85%–90% Is understood as comprising two interlaced sequences one consists of 57La, 58Ce,Īnd 64Gd, which have electronic configuration (4 f n−1)(5d1)(6s2), and the remainingĪtoms have configuration (4 f n)(6s2). Increases and decreases with rising nuclear charge from 57La to 70Yb. The value of rear decreases from 55Cs to 56Ba and undergoes Values of rear are about 50% larger than the mean radius of the outermost occupied Is defined as the distance from the nucleus at which the magnitude of the electricįield is equal to that in He at one half of the equilibrium bond length of He2. We consider, for atoms from 55Cs to 80Hg, the effective atomic radius (rear), which


 0 kommentar(er)
0 kommentar(er)
